Why it is important to understand the implications of combining dissimilar metals.
You might have heard the terms, “galvanic reaction”, “galvanic corrosion”, “galvanic series” dished out in the field. What these refer to are electrical / chemical reactions that occur when two dissimilar metals make contact in the presence of an electrolyte (in this instance, water). This results in corrosion, rust, reactions and weakness which could spell disaster for metal roofs and cladding throughout the building envelope.

In other words, a lot can go wrong when architectural metals are not properly placed. They don’t even need to be physically touching each other. Rainwater / moist air etc. when combined with other reactive materials found in the environment (electrolyte) speed the corrosion of and cause adverse reactions with dissimilar metals when runoff from one material meets that of the other. In architecture, and mostly from our standpoint on roofing and building envelope, we see failure because a more reactive material sits above a more sacrificial one.
In other cases, metal directly in contact with another metal such as galvanized fasteners used to secure copper cladding material will certainly result in weakening and eventual failure.
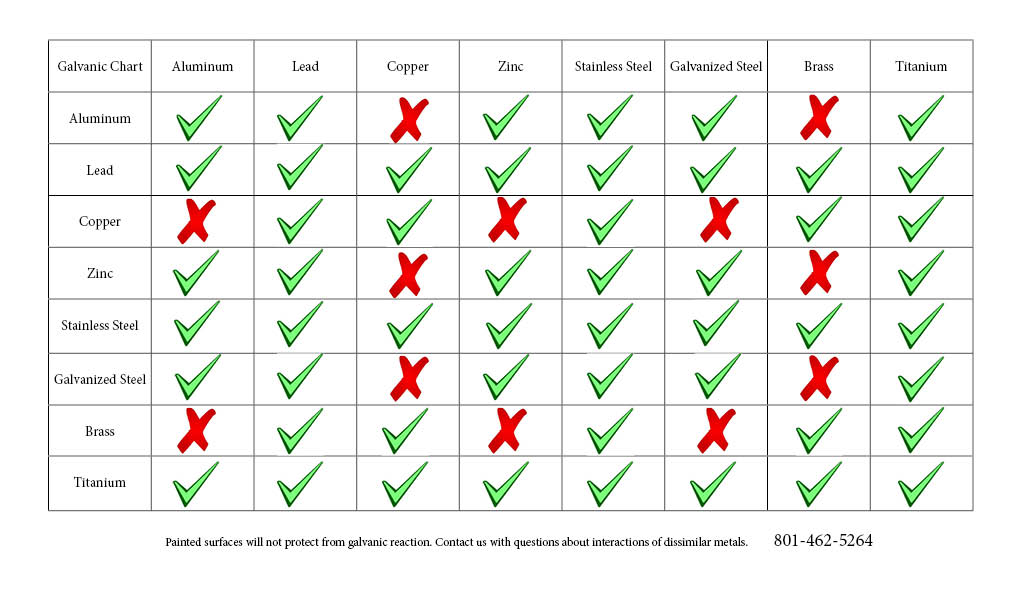
This said, certain dissimilar metals can be effectively commingled for design and fastening. From the standpoint of a chemist / metallurgist, this chart might be a bit too simplistic, but for our purposes, it proves a handy reference. Citing most of the roofing and cladding and architectural metals in the alloys most commonly encountered, this should clear up most questions about which metals can be safely combined:
Electric names:
Professor Luigi Galvani discovered the physiological action of electricity and demonstrated the existence of natural electric current in animal tissue. Volta, a professor of experimental physics in the University of Pavia, was among the first scientists who repeated and verified Galvani’s experiments. Though the two scientists disagreed respectfully on a number of issues, Volta coined the term “Galvanism” for a direct current of electricity produced by chemical action.
There is a lot to discuss and arrange when factoring for galvanic issues. We’ll gladly help you plan to ensure your building is built safely and correctly to effect assurance and permanence.
Call today: 801-462-5264